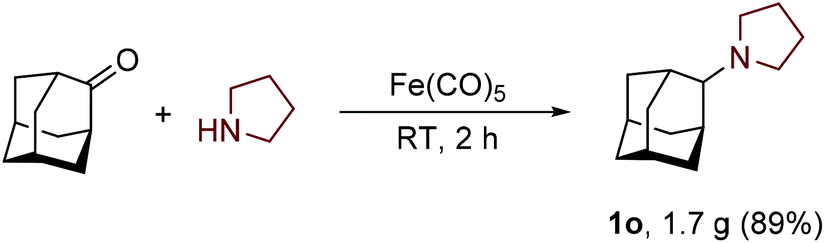Publications
2025
Direct synthesis of amides from nitroarenes and carboxylic acids via CO-mediated reduction
J. Cat. 2025, 445, 116042, Impact Factor: 6.615
Sodium Hypophosphite Assisted Ruthenium Catalyzed Reductive Amination under Mild Conditions
Org. Lett. 2025, ASAP, Impact Factor: 5.051
Aldehydes as reducing agents: Reductive alkylation of ketones
J. Cat. 2026, 116495, Impact Factor: 6.615
2024
Cyano-Fluorosulfonylation of Unactivated Alkenes by Photoredox and Copper Dual Catalysis
Org. Lett.. 2024, 26, 42, 9132–9137, Impact Factor: 4.885
Application of transition metal fluorides in catalysis
Coord. Chem. Rev., 2024, 519, 216114. Impact Factor: 20.63
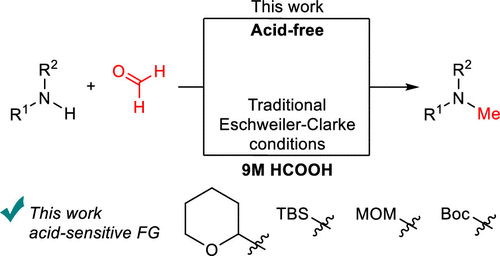
Simplified Version of the Eschweiler–Clarke Reaction
J. Org. Chem. 2024, 89, 5, 3580–3584 Impact Factor: 3.458
This article, 18 days after publication, was in the Top 10 JOC Most Read articles in 30 days.
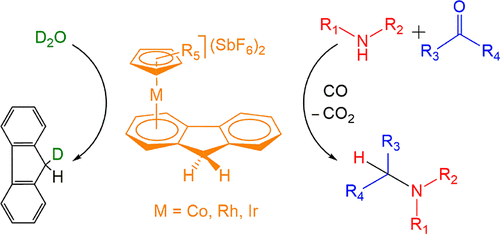
Air-Stable Arene Manganese Complexes as Catalysts for the Syngas-Assisted Direct Reductive Amination, Cyanation of Aldehyde, and CO2 Fixation by Epoxide with High Functional Groups Tolerance
J. Org. Chem. 2024, 89, 14, 10338–10343. Impact Factor: 3.458
Peculiarities of fluoride activation of porphyrin and phthalocyanine catalysts by the example of zirconium and hafnium in the production of cyclic carbonates
Mol. Cat., 2023, 549,113432, Impact Factor: 5.062
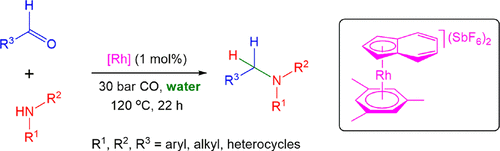
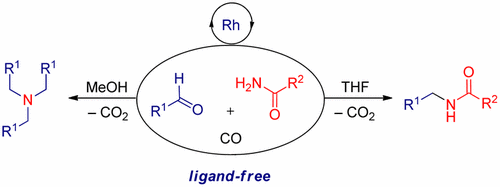
Syngas as a synergistic reducing agent for selective reductive amination—a mild route to bioactive amines
New J. Chem., 2023, 47, 10514-10518, Impact Factor: 3.925
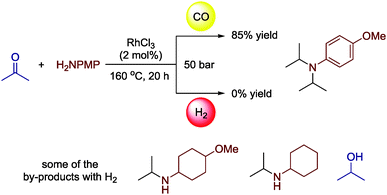
Nitrogen ligand influence on the CO-assisted ruthenium-catalyzed reductive amination
Mend. Commun., 2023; 33 (2), 174-176, Impact Factor: 1.694
Reductive α-alkylation of ketones with aldehydes at atmospheric pressure of carbon monoxide: the effect of fluoride activation in ruthenium catalysis
Mend. Commun., 2023; 33 (1), 17-20, Impact Factor: 1.694
Sodium hypophosphite mediated reductive amination of carbonyl compounds with N, N-dialkylformamides
2022
Borrowing Hydrogen Amination Reactions: A Complex Analysis of Trends and Correlations of the Various Reaction Parameters
Syngas Instead of Hydrogen Gas as a Reducing Agent─ A Strategy To Improve the Selectivity and Efficiency of Organometallic Catalysts
Sodium Hypophosphite as a Bulk and Environmentally Friendly Reducing Agent in the Reductive Amination
Org. Lett. 2022, Articles ASAP, Impact Factor: 6.005
Enhancing the efficiency of the ruthenium catalysts in the reductive amination without an external hydrogen source
J. Catal., 2021; 405, 2022, 404-409, Impact Factor: 7.92
Borrowing hydrogen amination: Whether a catalyst is required?
J. Catal., 2022; 413, 2022, 1070-1076, Impact Factor: 7.92
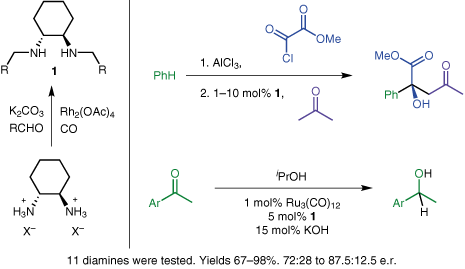
Asymmetric cyclopropanation of electron-rich alkenes by the racemic diene rhodium catalyst: the chiral poisoning approach
Fluoride Additive as a Simple Tool to Qualitatively Improve Performance of Nickel-Catalyzed Asymmetric Michael Addition of Malonates to Nitroolefins
Carbon monoxide-driven osmium catalyzed reductive amination harvesting WGSR power
Catal. Sci. Technol., 2021,11, 4922-4930, Impact Factor: 6.119
Reductive Aldol-type Reactions in the Synthesis of Pharmaceuticals
Frontispiece: Reductive Aldol-type Reactions in the Synthesis of Pharmaceuticals
Phosphine ligands in the ruthenium-catalyzed reductive amination without an external hydrogen source
J. Organomet. Chem., 2021; 941, 121806, Impact Factor: 2.369
Hayashi ligand-based rhodium complex in carbon monoxide and molecular hydrogen-assisted reductive amination
Mend. Commun., 2021; 31 (6), 781-783, Impact Factor: 1.694
Anthracene–rhodium complexes with metal coordination at the central ring – a new class of catalysts for reductive amination
Org. Biomol. Chem., 2019, 17, 1, 83-87, Impact Factor: 3.876